
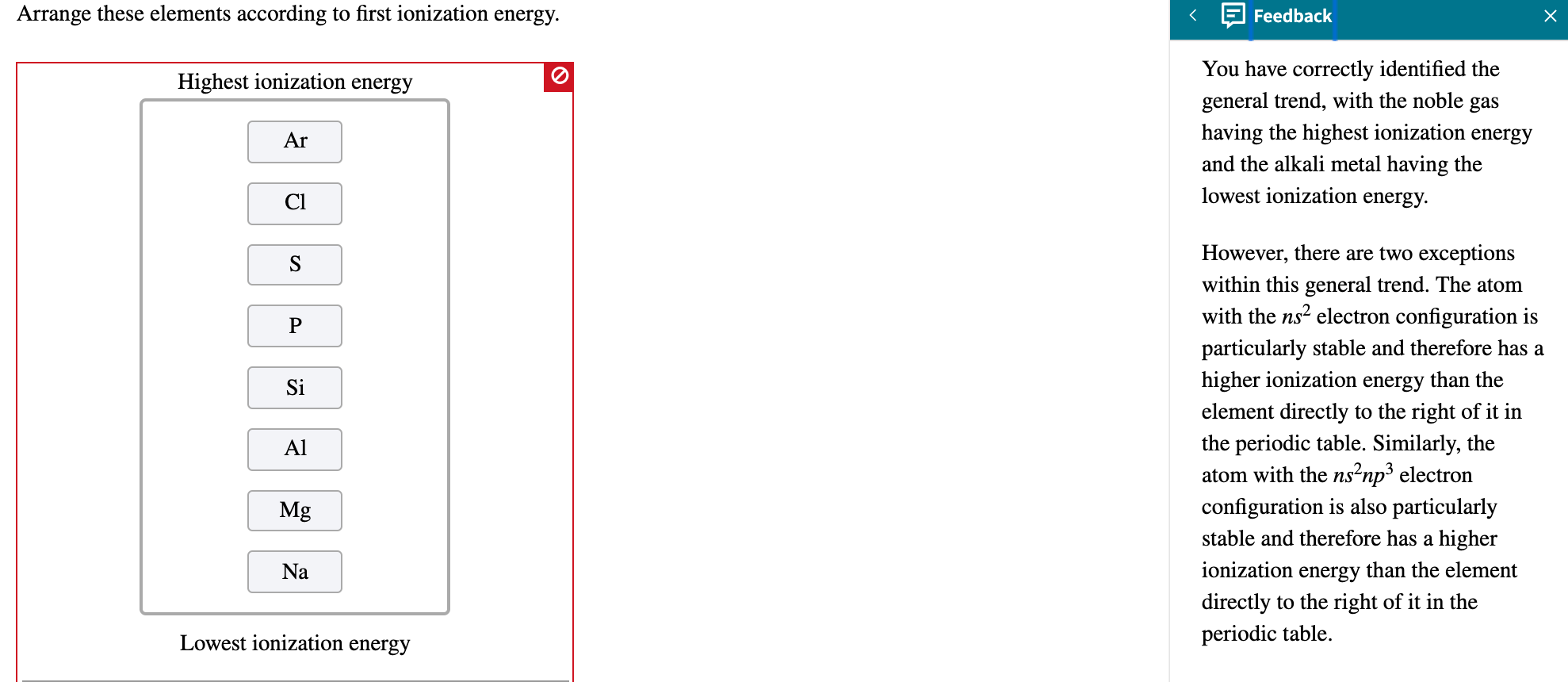
A Na < K < Mg < Rb B K < Na < Mg < Rb C Mg < Na < K < Rb D Rb < K < Mg < Na Medium Solution Verified by Toppr Correct option is C) The increasing order of atomic radius is Mg
All have a filled 1 s 2 inner shell, but as we go from left to right across the row, the nuclear charge increases from +3 to +10. The atoms in the second row of the periodic table (Li through Ne) illustrate the effect of electron shielding. When we are moving down the group Dominic the radius atomic radius increases. Look up chemical element names, symbols, atomic. So as we know that then to be loathing down the group. Interactive periodic table with up-to-date element property data collected from authoritative sources.

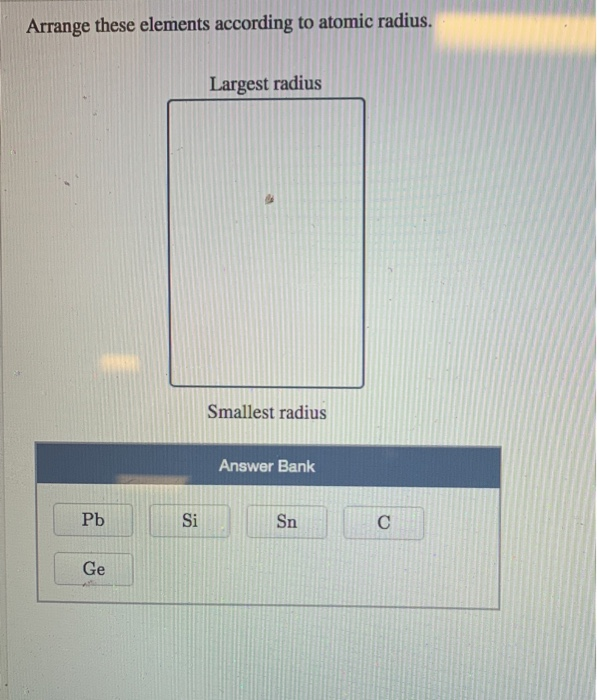
Largest radius Smallest radius Answer Bank Li Lit Li Arrange the atom and ions from largest to smallest radius. Largest radius Smallest radius Answer Bank Be Mg Mg Rb Ca Arrange the atom and ions according to radius. And we have to arrange them in increasing order of atomic radius. Largest Smallest Answer Bank oxygen silicon calcium barium Arrange these elements according to atomic radius. The greater the effective nuclear charge, the more strongly the outermost electrons are attracted to the nucleus and the smaller the atomic radius.Ītomic radii decrease from left to right across a row and increase from top to bottom down a column. That is oxygen, sulfur, selenium, thallium. For all elements except H, the effective nuclear charge is always less than the actual nuclear charge because of shielding effects. \( \newcommand\)) experienced by electrons in the outermost orbitals of the elements.


 0 kommentar(er)
0 kommentar(er)
